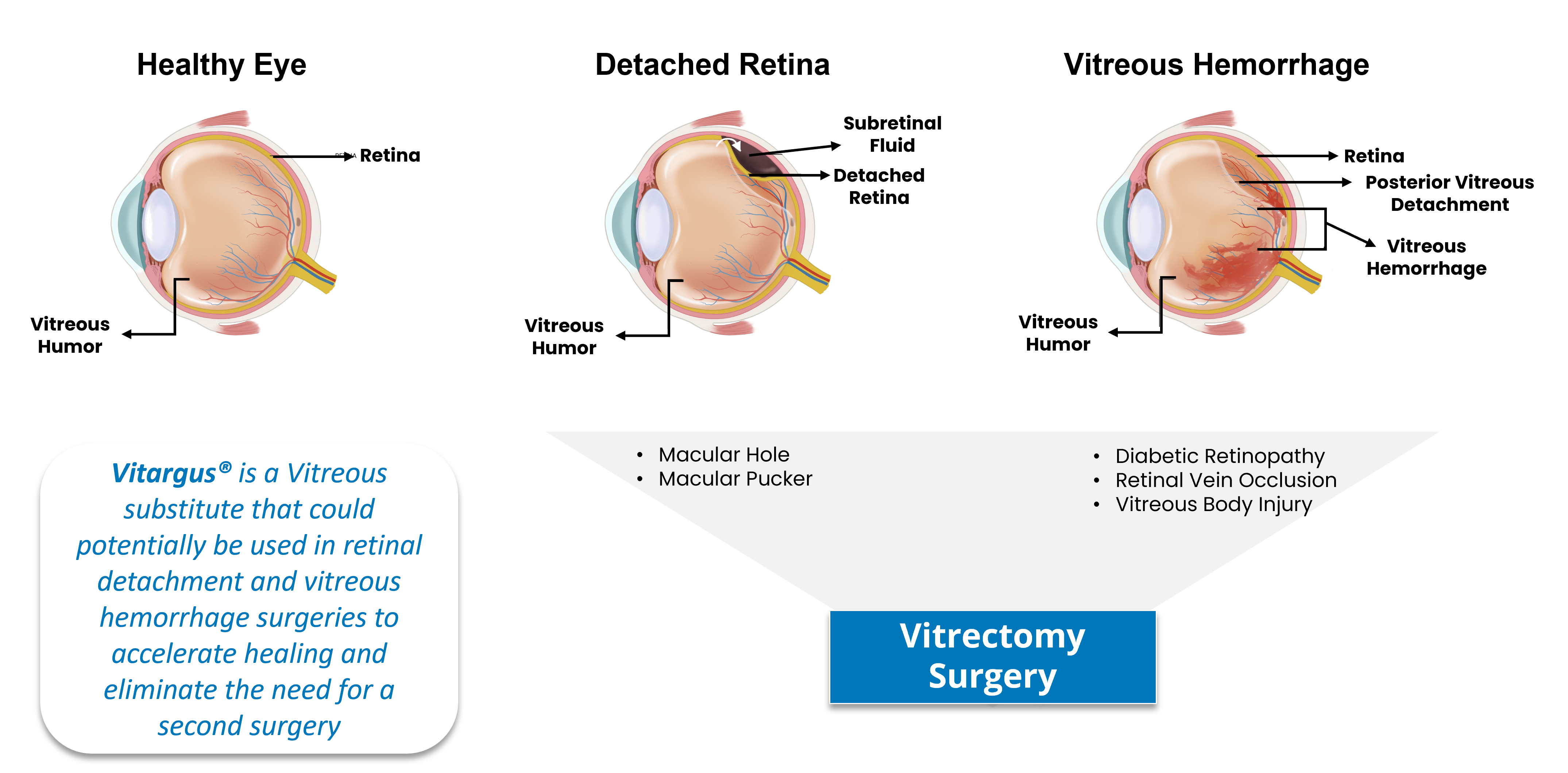
Vitargus represents a significant breakthrough in the field of ophthalmology. Early clinical studies indicate that Vitargus® has unique properties that could eliminate the need for patients to remain face-down after retinal detachment and vitreous hemorrhage surgery, as well as significantly improve patient comfort and visual acuity during the surgical recovery period compared to currently available products.
ForSeeCon is dedicated to advancing eye healthcare worldwide. With a steadfast commitment to research, development, manufacturing, and sales and marketing of pharmaceutical products, we proudly support the eye health of approximately 50 million people globally.
Our expertise in ophthalmology drives us to continuously innovate and address the unmet needs of patients and the ophthalmic medical community. Through our expanding portfolio, now available in over 30 countries, we strive to make a meaningful impact on the lives of those affected by a wide range of eye diseases.

Our approach is comprehensive and collaborative. We prioritize research and development, partnering closely with local clinics to ensure our products meet the highest standards of efficacy and safety. From production to sales and marketing, every aspect of our operations is guided by our dedication to excellence and our unwavering commitment to improving eye health worldwide.
"We have a strong commitment to innovation" says Jerry Chang, CEO of ForSeeCon. We collaborate with leading development partners in Australia and Thailand, leveraging their expertise to refine and advance our products. This commitment to innovation is reinforced by our extensive patent portfolio, ensuring protection for our intellectual property across key markets worldwide.

The global retinal detachment disorder market continues to grow, with millions of patients worldwide undergoing eye surgery each year. At ForSeeCon, we recognize the importance of providing advanced solutions to meet this increasing demand. Vitargus offers a promising alternative to traditional vitreous substitutes, improving patient outcomes and enhancing surgical procedures.

Our journey began with the first in-human study of Vitargus in 2018, marking a significant milestone in our quest to transform eye healthcare. Through rigorous clinical trials and meticulous research, we have demonstrated the safety and efficacy of Vitargus, with no evidence of treatment-related toxicity. This commitment to excellence underscores our dedication to providing healthcare professionals and patients with reliable and effective solutions.

ForSeeCon welcomes partnerships and collaborations with healthcare professionals, institutions, and organizations that share our vision for advancing eye healthcare. Together, we can make a meaningful difference in the lives of patients around the world, ensuring access to innovative treatments and improving outcomes for those in need.